ASSOCIATED PRESS by Jpnathan Paye-Layleh March 22, 2015
MONROVIA, Liberia — Liberians are overcoming their fears of Ebola to volunteer for a vaccine trial...
One year after the World Health Organization declared the Ebola outbreak, vaccine trials are under way in Liberia and Guinea. Sierra Leone will start a trial later this month.
In Liberia, scientists have fanned out across the country to explain the studies and reduce the fear and confusion that have stymied efforts to contain Ebola.
Dr. Stephen Kennedy, the Liberian lead investigator for the study, was among the first people to volunteer for the vaccine trial, getting his injection in front of the media. Similarly, in Guinea, authorities started the study by injecting a series of prominent officials, including the head of the country's Ebola response.
The outreach worked in Liberia, where more than 700 people have volunteered, well beyond the 600 required, according to Kennedy.
Read complete story.
http://news.yahoo.com/liberians-overcome-fear-volunteer-ebola-vaccine-trial-120848405.html






 These are western lowland gorillas, one of the great ape species threatened by Ebola. Credit: Copyright 2012 Chris Whittier
These are western lowland gorillas, one of the great ape species threatened by Ebola. Credit: Copyright 2012 Chris Whittier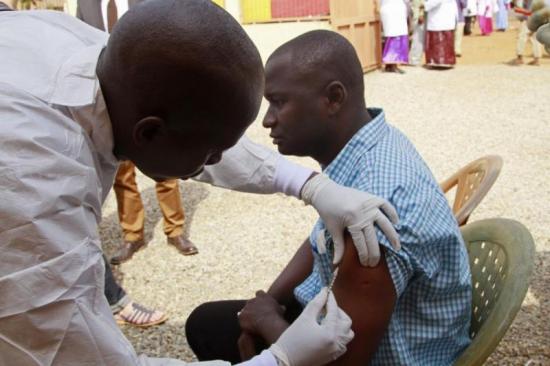
Recent Comments