CENTER FOR INFECTIOUS DISEASE AND POLICY by Lisa Schnirring Feb. 13, 2015
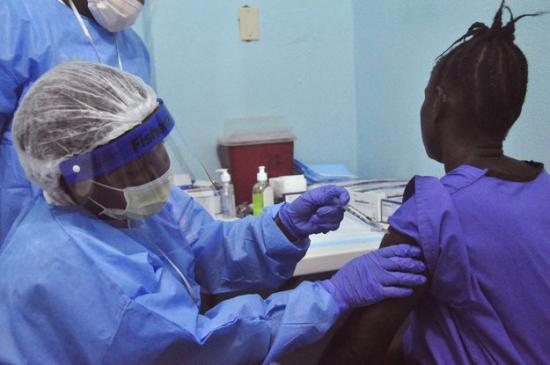
Novavax yesterday announced the launch of the first human trial of its recombinant Ebola vaccine, which will make it the fourth candidate vaccine to be tested in phase 1 trials.
 Novavax's product is a glycoprotein recombinant nanoparticle vaccine adjuvanted with Matrix M (Ebola GP) to boost immune response. Conducted in Australia, the study will test the safety and immunogenicity of the vaccine, with and without the adjuvant, in 230 healthy adults ages 18 to 50. Subjects will be given two intramuscular injections 3 weeks apart....
Novavax's product is a glycoprotein recombinant nanoparticle vaccine adjuvanted with Matrix M (Ebola GP) to boost immune response. Conducted in Australia, the study will test the safety and immunogenicity of the vaccine, with and without the adjuvant, in 230 healthy adults ages 18 to 50. Subjects will be given two intramuscular injections 3 weeks apart....
Three other Ebola vaccines are in clinical trials. Phase 2 and 3 studies of the two vaccines that are furthest along in trials got under way in Liberia at the end of January. They include two vector virus vaccines, ChAd3, developed by the National Institutes of Health (NIH) and GlaxoSmithKline (GSK), and VSV-EBOV, developed by the Canadian government and licensed by NewLink Genetics and Merck.
A phase 1 trial of a prime-boost Ebola vaccine regimen from Johnson & Johnson launched in early January in the United Kingdom.




 Novavax's product is a glycoprotein recombinant nanoparticle vaccine adjuvanted with Matrix M (Ebola GP) to boost immune response. Conducted in Australia, the study will test the safety and immunogenicity of the vaccine, with and without the adjuvant, in 230 healthy adults ages 18 to 50. Subjects will be given two intramuscular injections 3 weeks apart....
Novavax's product is a glycoprotein recombinant nanoparticle vaccine adjuvanted with Matrix M (Ebola GP) to boost immune response. Conducted in Australia, the study will test the safety and immunogenicity of the vaccine, with and without the adjuvant, in 230 healthy adults ages 18 to 50. Subjects will be given two intramuscular injections 3 weeks apart....
Recent Comments