You are here
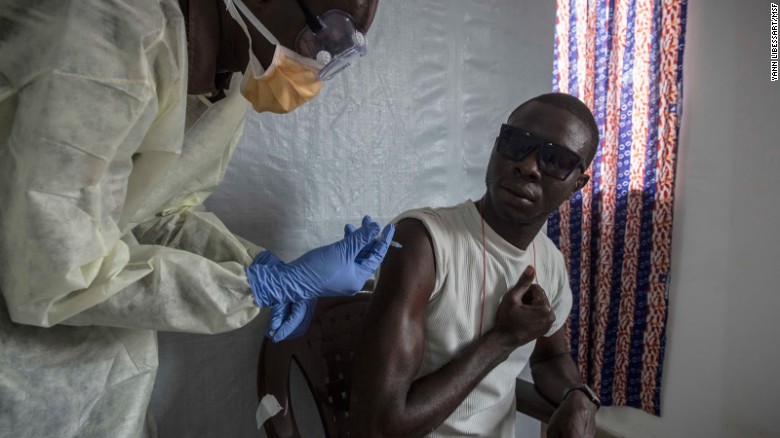
On March 23, 2015, Medecins Sans Frontieres (Doctors Without Borders) together with the World Health Organization started a clinical trial in Guinea to test the safety, efficacy and capacity substance to provoke an immune response of the anti-Ebola vaccine rVSV-EBOV.
cnn.com - by Laura Smith-Spark - July 31, 2015
(CNN) A newly developed vaccine against the deadly Ebola virus is "highly effective" and could help prevent its spread in the current and future outbreaks, the World Health Organization said Friday.
Trials of the single-dose VSV-EBOV vaccine began in March in Guinea -- one of three West African nations at the center of the outbreak -- and have shown such promise that this week it was decided to extend immediate vaccination to "all people at risk" after close contact with an infected person, a WHO news release said.
"This is an extremely promising development," said Dr. Margaret Chan, the body's director general.
CLICK HERE - The Lancet - An Ebola vaccine: first results and promising opportunities



Comments
A New Ebola Vaccine Shows Promise - Good Reason for Caution
vox.com - Julia Belluz - August 3, 2015
Exactly a year ago, West Africa was battling the most devastating Ebola outbreak in human history.
Now, after nearly 30,000 cases and 12,000 deaths, there's finally some good news: Researchers think they might have a vaccine that can protect people from the deadly virus. . . .
. . . But there is reason to be cautious about the findings.
(READ COMPLETE ARTICLE)
Conversation: John-Arne Røttingen, Ebola Vaccine Co-Investigator
allafrica.com - August 5, 2015
INTERVIEW
John-Arne Røttingen, the director of the Division of Infectious Disease Control at the Norwegian Institute of Public Health and adjunct professor of Global Health and Population at Harvard University, is one of the co-authors of the successful rVSV-ZEBOV Ebola vaccine trial. He worked alongside the Wellcome Trust, the government of Canada, MSF, the London School of Hygiene and Tropical Medicine and manufacturers Merck and NewLink.
(READ COMPLETE INTERVIEW)
Ebola Vaccine Highly Efficacious in a Ring Vaccination Trial
jwatch.org - NEJM Journal Watch - August 5, 2015
Mary E. Wilson, MD reviewing Henao-Restrepo AM et al. Lancet 2015 Jul 31.
A single dose of a live candidate Ebola vaccine prevented all infections among contacts ≥6 days after vaccination
Several candidate Ebola vaccines have been developed. One — a recombinant, vesicular stomatitis virus–based preparation expressing the glycoprotein of a Zaire Ebolavirus (rVSV-ZEBOV; NEJM JW Infect Dis May 2015 and N Engl J Med 2015 Apr 1; [e-pub]) — causes transient systemic infection and induces rapid immune response against Ebola virus surface protein.
In an open-label, cluster-randomized, ring vaccination trial in Guinea in 2015, investigators administered a single intramuscular injection of rVSV-ZEBOV to the contact networks of patients with confirmed Ebola virus disease (EVD) immediately after the identified exposure or 21 days later. Nonpregnant adults with contact during the preceding 21 days were eligible. The number of individuals per cluster was 58 to 100.
Interim analysis of infections up to July 20, 2015, has now been completed. Among 2014 vaccinees (48 clusters) in the immediate-vaccination group, no cases of EVD were observed with onset of symptoms ≥10 days after randomization, for an efficacy of 100%. In the delayed-vaccination group (2380 eligible — but not necessarily vaccinated —people, 42 clusters), 16 cases of EVD occurred. No vaccinees in either group developed EVD ≥6 days postimmunization.
Among all 5415 vaccination-eligible contacts and contacts of contacts (vaccination rates, 42%–49%), 6 immediate-group participants and 16 delayed-group participants developed EVD, for an estimated vaccine effectiveness of 75%.
Only one serious vaccine-related adverse event (fever, followed by full recovery) has been reported.
(READ COMPLETE ARTICLE)
A single dose of a live candidate Ebola vaccine prevented all infections among contacts ≥6 days after vaccination
Several candidate Ebola vaccines have been developed. One — a recombinant, vesicular stomatitis virus–based preparation expressing the glycoprotein of a Zaire Ebolavirus (rVSV-ZEBOV; NEJM JW Infect Dis May 2015 and N Engl J Med 2015 Apr 1; [e-pub]) — causes transient systemic infection and induces rapid immune response against Ebola virus surface protein.
In an open-label, cluster-randomized, ring vaccination trial in Guinea in 2015, investigators administered a single intramuscular injection of rVSV-ZEBOV to the contact networks of patients with confirmed Ebola virus disease (EVD) immediately after the identified exposure or 21 days later. Nonpregnant adults with contact during the preceding 21 days were eligible. The number of individuals per cluster was 58 to 100.
Interim analysis of infections up to July 20, 2015, has now been completed. Among 2014 vaccinees (48 clusters) in the immediate-vaccination group, no cases of EVD were observed with onset of symptoms ≥10 days after randomization, for an efficacy of 100%. In the delayed-vaccination group (2380 eligible — but not necessarily vaccinated —people, 42 clusters), 16 cases of EVD occurred. No vaccinees in either group developed EVD ≥6 days postimmunization.
Among all 5415 vaccination-eligible contacts and contacts of contacts (vaccination rates, 42%–49%), 6 immediate-group participants and 16 delayed-group participants developed EVD, for an estimated vaccine effectiveness of 75%.
Only one serious vaccine-related adverse event (fever, followed by full recovery) has been reported.
- See more at: http://www.jwatch.org/na38703/2015/08/05/ebola-vaccine-highly-efficacious-ring-vaccination-trial#sthash.mLIw0DLV.dpuf
A single dose of a live candidate Ebola vaccine prevented all infections among contacts ≥6 days after vaccination
Several candidate Ebola vaccines have been developed. One — a recombinant, vesicular stomatitis virus–based preparation expressing the glycoprotein of a Zaire Ebolavirus (rVSV-ZEBOV; NEJM JW Infect Dis May 2015 and N Engl J Med 2015 Apr 1; [e-pub]) — causes transient systemic infection and induces rapid immune response against Ebola virus surface protein.
In an open-label, cluster-randomized, ring vaccination trial in Guinea in 2015, investigators administered a single intramuscular injection of rVSV-ZEBOV to the contact networks of patients with confirmed Ebola virus disease (EVD) immediately after the identified exposure or 21 days later. Nonpregnant adults with contact during the preceding 21 days were eligible. The number of individuals per cluster was 58 to 100.
Interim analysis of infections up to July 20, 2015, has now been completed. Among 2014 vaccinees (48 clusters) in the immediate-vaccination group, no cases of EVD were observed with onset of symptoms ≥10 days after randomization, for an efficacy of 100%. In the delayed-vaccination group (2380 eligible — but not necessarily vaccinated —people, 42 clusters), 16 cases of EVD occurred. No vaccinees in either group developed EVD ≥6 days postimmunization.
Among all 5415 vaccination-eligible contacts and contacts of contacts (vaccination rates, 42%–49%), 6 immediate-group participants and 16 delayed-group participants developed EVD, for an estimated vaccine effectiveness of 75%.
Only one serious vaccine-related adverse event (fever, followed by full recovery) has been reported.
- See more at: http://www.jwatch.org/na38703/2015/08/05/ebola-vaccine-highly-efficacious-ring-vaccination-trial#sthash.mLIw0DLV.dpuf
A single dose of a live candidate Ebola vaccine prevented all infections among contacts ≥6 days after vaccination
Several candidate Ebola vaccines have been developed. One — a recombinant, vesicular stomatitis virus–based preparation expressing the glycoprotein of a Zaire Ebolavirus (rVSV-ZEBOV; NEJM JW Infect Dis May 2015 and N Engl J Med 2015 Apr 1; [e-pub]) — causes transient systemic infection and induces rapid immune response against Ebola virus surface protein.
In an open-label, cluster-randomized, ring vaccination trial in Guinea in 2015, investigators administered a single intramuscular injection of rVSV-ZEBOV to the contact networks of patients with confirmed Ebola virus disease (EVD) immediately after the identified exposure or 21 days later. Nonpregnant adults with contact during the preceding 21 days were eligible. The number of individuals per cluster was 58 to 100.
Interim analysis of infections up to July 20, 2015, has now been completed. Among 2014 vaccinees (48 clusters) in the immediate-vaccination group, no cases of EVD were observed with onset of symptoms ≥10 days after randomization, for an efficacy of 100%. In the delayed-vaccination group (2380 eligible — but not necessarily vaccinated —people, 42 clusters), 16 cases of EVD occurred. No vaccinees in either group developed EVD ≥6 days postimmunization.
Among all 5415 vaccination-eligible contacts and contacts of contacts (vaccination rates, 42%–49%), 6 immediate-group participants and 16 delayed-group participants developed EVD, for an estimated vaccine effectiveness of 75%.
Only one serious vaccine-related adverse event (fever, followed by full recovery) has been reported.
- See more at: http://www.jwatch.org/na38703/2015/08/05/ebola-vaccine-highly-efficacious-ring-vaccination-trial#sthash.mLIw0DLV.dpuf