You are here
The company producing the new Ebola treatment for an FDA-approved test suddenly pulled out of Liberia, leaving researchers confused.
BUZZFEED by Hayes Brown Feb. 13, 2015
...An FDA-approved trial of the drug brincidofovir, meant to treat rather than prevent Ebola, had already begun in Liberia’s capital of Monrovia when Chimerix, the company that produced the drug, pulled out of the trial at the end of January. The clinical trial partners decided to end the trial on Feb. 3.
Peter Horby, who led the University of Oxford research team conducting the study, called the drug company’s decision “a bit abrupt.”
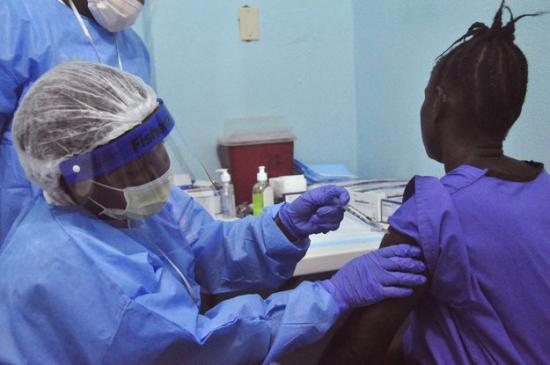
A woman is injected by a health care worker as she takes part in an Ebola virus vaccine trial in Monrovia Abbas Dulleh / Via AP
Horby said the research consortium — which included the trial funder, an independent British medical research organization called the Wellcome Trust, and Doctors Without Borders, whose clinics were being used as research sites — had discussed adding additional sites to the study in the event that case numbers at its first site dropped too low.
Horby said the research team had received and stored enough doses of brincidofovir in Liberia to scale up to additional sites but that the drugs would have to be destroyed following the Chimerix withdrawal.
Read complete story.
http://www.buzzfeed.com/hayesbrown/nobody-is-sure-why-a-promising-ebola-drug-trial-ended#.caGzQJXrG



Recent Comments