You are here
Pfizer submits data to FDA for use of its Covid vaccine for children ages 5 to 11
Primary tabs
Tue, 2021-09-28 09:21 — mike kraft
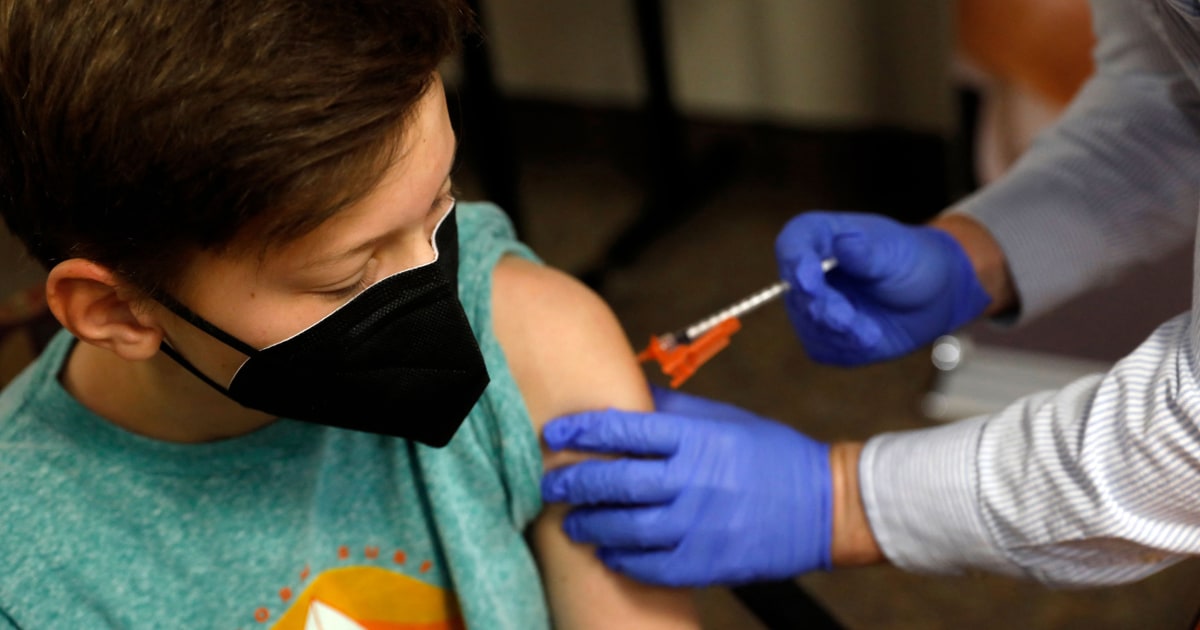 Pfizer submits data to FDA, seeking to use its Covid vaccine for children ages 5 to 11 These younger kids could be vaccinated by Halloween, Pfizer CEO says. NBC News
Pfizer submits data to FDA, seeking to use its Covid vaccine for children ages 5 to 11 These younger kids could be vaccinated by Halloween, Pfizer CEO says. NBC News
 Pfizer submits data to FDA, seeking to use its Covid vaccine for children ages 5 to 11 These younger kids could be vaccinated by Halloween, Pfizer CEO says. NBC News
Pfizer submits data to FDA, seeking to use its Covid vaccine for children ages 5 to 11 These younger kids could be vaccinated by Halloween, Pfizer CEO says. NBC News Pfizer-BioNTech submitted data to the Food and Drug Administration to clear its Covid-19 vaccine for use in children ages 5 to 11, officials announced Tuesday.
The FDA is expected to take at least several weeks to analyze data collected in a trial that included more than 2,000 children before it would grant emergency use authorization.
A formal submission to request emergency use authorization of the companies’ vaccine is expected to follow in the coming weeks, officials said in a release. ...
Country / Region Tags:
General Topic Tags:
Problem, Solution, SitRep, or ?:
Groups this Group Post belongs to:



Recent Comments